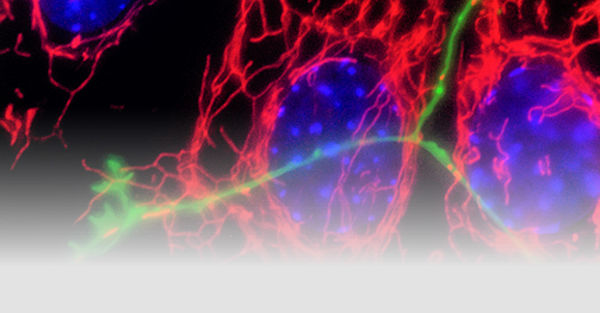
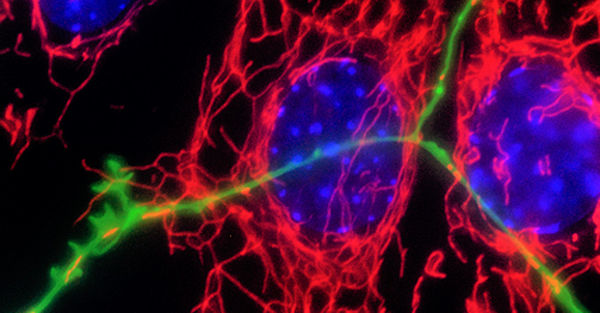
Neurodegenerative disorders are associated in a proportion of cases with genetic risk and gene mutations. However, the vast majority of cases of Parkinson’s disease are sporadic and the disease is altogether heterogeneous in symptoms and pathology. The Fitzgerald group aims to understand the molecular mechanisms underlying neurodegeneration using genetic forms of the disease as an entry point and a focus on the role of mitochondria.
Mitochondria are crucial organelles that produce energy and perform many other functions needed for central metabolism and cell signalling. Neurons use a lot of energy and rely on tight control for efficiency and to reduce oxidative burden. Mitochondrial dysfunction is a phenomenon that traverses all neurodegenerative diseases and may be an important in explaining the selective vulnerability of certain brain cells. This is especially relevant in Parkinson’s disease, where the dopaminergic neurons die, because the mitochondria are a major source of oxidative stress and the location of dopamine degradation.
In Parkinson’s disease, the link between mitochondrial dysfunction and disease has been proven by the identification of environmental factors and disease genes which critically affect mitochondria. The outcome has been a large body of work depicting the role of mitochondrial dysfunction in Parkinson’s disease, yet the exact mechanisms underlying sporadic forms of PD are less defined.
We utilize several different model cell systems with focus on patient-derived cells. In close collaboration with the Hertie Institute biobank and the Neurology Clinics, we are currently working in primary human fibroblasts derived from patients and healthy individuals, induced pluripotent stem cells (iPSCs), small molecule neuronal progenitor cells (smNPCs) and mature dopaminergic neurons. We are also working with peripheral blood cells (PBMCs) from patients and healthy individuals.
We are performing mostly functional work focusing on biology, biochemistry and physiology. We have established several specialized techniques for monitoring mitochondrial health and have also recently established several protein biochemistry, imaging and flow cytometry methods for investigating the endosomal-lysosomal system.

The role of PINK1 in an iPSC-derived neuronal model of Parkinson’s disease
Anna Schaedler, Christine Bus (Alumni)
We are currently investigating the role of the Parkinson’s disease kinase PINK1 in human dopaminergic neurons derived from iPSCs and using gene editing technology to generate isogenic controls. With several established readouts to assess mitochondrial function following different stress conditions, we were able to identify distinct mitochondrial phenotypes, which suggest a role for PINK1 in mitochondrial quality control. Using these PINK1 deficient models and their isogenic controls we will now look for genetic and pharmacological modifiers to apply novel neuroprotective strategies.
Max Mattheuer
Mutations in the mitochondrial PTEN-induced putative kinase 1 (PINK1) are the second most common cause of recessive inherited Parkinson´s disease. The mechanism by which the loss of function mutation of PINK1 leads to the death of dopaminergic neurons in the mid brain remains elusive. Working with an iPSC derived model of dopaminergic neurons deficient in PINK1, we aim to gain further insights into the underlying mechanisms. In PINK1 knock out dopamine neurons no classical mitophagy after mitochondrial membrane depolarization was observed, while levels of autophagy and ROS production remained unchanged. The goal of the project is to find potential mechanisms of mitochondrial quality control/recycling in PINK1 knock out dopamine neurons in vitro.
The role of Miro1 in mitochondrial quality control
Lisa Schwarz
Mitochondria play a crucial role in several cellular mechanisms including energy supply, calcium homeostasis and apoptosis. While the matrix and mitochondrial inner membrane are well characterized, little is known about the mitochondrial outer membrane (MOM). The German Research Council research training group (RTG) MOMbrane aims to close this gap by to investigating the various functions of the MOM as well as its structure, regulation and biogenesis. The MOMbrane includes ten PhD students in different institutes in Tübingen, each focusing on a different aspect of the MOM. The project is carried out in collaboration with partner labs of the Weizmann institute in Rehovot (Israel).
Miro1 is located at the MOM and known to be responsible for mitochondrial transport. As part of the MOMbrane RTG, we aim to elucidate the function of Miro1 in mitochondrial turnover, metabolism, calcium signaling. It is known that one of the pathological hallmarks of Parkinson’s disease is altered mitochondrial function. We aim to better understand the biological roles of Miro1 and also its relevance to Parkinson’s disease by studying relevant disease models. To achieve this knowledge, we are introducing disease-associated and functional Miro1 variants in Miro1 in human induced pluripotent stem cells (iPSCs) using gene editing. Healthy and edited iPSCs can then be differentiated into other cell types including neurons.
The role of the endosomal-lysosomal system in atypical Parkinson’s disease/Corticobasal syndrome
Katharina Stegen (Alumni)
Dysfunction of the endosomal-lysosomal system is a phenomenon that traverses many neurodegenerative diseases including Alzheimer’s diseases, Parkinson’s disease and other rarer neurodegenerative diseases such as progressive supranuclear palsy (PSP) and corticobasal syndrome/degeneration (CBS/CBD). The endosomal lysosomal system is formed and driven by changes in pH and therefore the ion exchangers that reside in the membranous compartments of the endosomal lumen are critical for correct pH regulation. These exchangers are so crucial that major defects in them often cause severe mental retardation from birth. We are investigating defects that instead confer susceptibility to develop neurodegenerative diseases in later life. We are also investigating aspects of cellular trafficking involving the endosomal-lysosomal system and autophagy that could be involved in sporadic Parkinson’s disease.
Mitochondrial biomarkers in sporadic Parkinson’s disease
Julia Fitzgerald, Gerrit Machetanz, Anne Grünewald (LCSB, Université du Luxembourg)
Sporadic forms of Parkinson’s disease are the most common but they are difficult to model in the laboratory because there is no known gene mutation that can be introduced or corrected to generate isogenic controls. Statistically, large cohorts are needed to compare healthy individuals with sporadic Parkinson’s disease patients. This poses a problem because culturing patient-derived cell lines in parallel for such large cohorts requires specialist robotics, it is invasive for patients and is very expensive. To overcome this, we are collecting blood cells from a large cohort of Parkinson’s disease patients including sporadic and familial cases where mitochondrial proteins are affected. We will look for changes in mitochondria in these cells to try to identify subgroups of patients that may in future benefit from medicines that target mitochondrial dysfunction.

+49 (0)7071-
29-81971

2022
Schmidt S, Luecken MD, Trümbach D, Hembach S, Niedermeier KM, Wenck N, Pflügler K, Stautner C, Böttcher A, Lickert H, Ramirez-Suastegui C, Ahmad R, Ziller MJ, Fitzgerald JC, Ruf V, van de Berg WDJ, Jonker AJ, Gasser T, Winner B, Winkler J, Vogt Weisenhorn DM, Giesert F, Theis FJ & Wurst W. Primary cilia and SHH signaling impairments in human and mouse models of Parkinson’s disease. Nat Commun 2022; 13, 4819 PMID: 35974013
Arena G, Sharma K, Agyeah G, Krüger R, Grünewald A, Fitzgerald JC. Neurodegeneration and Neuroinflammation in Parkinson's Disease: a Self-Sustained Loop Curr Neurol Neurosci Rep 2022; Jun 8:1–14 PMID: 35674870
Harmuth T, Weber JJ, Zimmer AJ, Sowa AS, Schmidt J, Fitzgerald JC, Schöls L, Riess O, Hübener-Schmid J. Mitochondrial Dysfunction in Spinocerebellar Ataxia Type 3 Is Linked to VDAC1 Deubiquitination Int. J. Mol. Sci 2022; 23, 5933 PMID: 35682609
Schwarz L and Fitzgerald JC. Steady-State Levels of Miro1 Linked to Phosphorylation at Serine 156 and Mitochondrial Respiration in Dopaminergic Neurons Cells 2022; 11, no. 8: 1269. PMID: 35455950
Kakade P, Ojha H, Raimi OG, Kakade P, Ojha H, Raimi OG, Shaw A, Waddell AD, Ault JR, Burel S, Brockmann K, Kumar A, Ahangar MS, Krysztofinska EM, Macartney T, Bayliss R, Fitzgerald JC, Muqit MMK. Mapping of a N-terminal α-helix domain required for human PINK1 stabilization, Serine228 autophosphorylation and activation in cells. Open Biol. 2022;12(1):210264 PMID: 35042401
Schmidt S, Luecken MD, Trümbach D, Hembach S, Niedermeier KM, Wenck N, Pflügler K, Stautner C, Böttcher A, Lickert H, Ramirez-Suastegui C, Ahmad R, Ziller MJ, Fitzgerald JC, Ruf V, van de Berg WDJ, Jonker AJ, Gasser T, Winner B, Winkler J, Vogt Weisenhorn DM, Giesert F, Theis FJ & Wurst W. Primary cilia and SHH signaling impairments in human and mouse models of Parkinson’s disease. Nat Commun 2022; 13, 4819 PMID: 35974013
Arena G, Sharma K, Agyeah G, Krüger R, Grünewald A, Fitzgerald JC. Neurodegeneration and Neuroinflammation in Parkinson's Disease: a Self-Sustained Loop Curr Neurol Neurosci Rep 2022; Jun 8:1–14 PMID: 35674870
Harmuth T, Weber JJ, Zimmer AJ, Sowa AS, Schmidt J, Fitzgerald JC, Schöls L, Riess O, Hübener-Schmid J. Mitochondrial Dysfunction in Spinocerebellar Ataxia Type 3 Is Linked to VDAC1 Deubiquitination Int. J. Mol. Sci 2022; 23, 5933 PMID: 35682609
Schwarz L and Fitzgerald JC. Steady-State Levels of Miro1 Linked to Phosphorylation at Serine 156 and Mitochondrial Respiration in Dopaminergic Neurons Cells 2022; 11, no. 8: 1269. PMID: 35455950
Kakade P, Ojha H, Raimi OG, Kakade P, Ojha H, Raimi OG, Shaw A, Waddell AD, Ault JR, Burel S, Brockmann K, Kumar A, Ahangar MS, Krysztofinska EM, Macartney T, Bayliss R, Fitzgerald JC, Muqit MMK. Mapping of a N-terminal α-helix domain required for human PINK1 stabilization, Serine228 autophosphorylation and activation in cells. Open Biol. 2022;12(1):210264 PMID: 35042401
2021
Schwarz L, Casadei N and Fitzgerald JC. Generation of R272Q, S156A and K572R RHOT1/Miro1 point mutations in iPSCs from a healthy individual using FACS-assisted CRISPR/Cas9 genome editing. Stem Cell Res. 2021 25(55), 102469. PMID: 34359002
Brown SJ, Boussaad I, Jazaro J, Fitzgerald JC, Antony P, Keatinge M, Blechman, J, Schwamborn J, Krüger R, Placzek M, Bandmann O. PINK1 deficiency impairs adult neurogenesis of dopaminergic neurons. Sci Rep 2021; 11, 6617. PMID: 33758225
Körner A, Bernard A, Fitzgerald JC, Alarcon-Barrerer JC, Kostidis S, Kaussen T, Giera M & Mirakaj V. Sema7A is crucial for resolution of severe inflammation. PNAS 2021; 118, e2017527118. PMID: 33637648
2020
Bus C, Zizmare L, Feldkaemper M, Geisler S, Zarani M, Schaedler A, Klose F, Admard, J, Mageean CJ, Arena J, Fallier-Becker P, Ugun-Klusek A, Maruszczak K, Kapolou K, Schmid B, Rapaport D, Ueffing M, Casadei N, Krüger R, Gasser T, Vogt-Weisenhorn D, Kahle PJ, Trautwein C, Gloeckner CJ and Fitzgerald JC. Human Dopaminergic Neurons Lacking PINK1 Exhibit Disrupted Dopamine Metabolism Related to Vitamin B6 Co-Factors. iScience 2020; 23, 12, 101797. PMID: 33299968
2019
Hertlein V, Flores-Romero H, Das KK, Fischer S, Heunemann M, Calleja-Felipe M, Knafo S, Hipp K, Harter K, Fitzgerald JC, García-Sáez AJ. MERLIN: a novel BRET-based proximity biosensor for studying mitochondria-ER contact sites. Life Sci Alliance 2019; Dec 9;3(1). PMID: 31818884
Grossmann D, Berenguer-Escuder C, Bellet M, Scheibner D, Bohler J, Massart F, Rapaport D, Skupin A, Fouquier d'Hérouël A, Sharma M, Ghelfi J, Raković A, Lichtner P, Antony P, Glaab E, May P, Dimmer K, Fitzgerald JC, Grünewald A, Krüger R. Mutations in RHOT1 Disrupt Endoplasmic Reticulum-Mitochondria Contact Sites Interfering with Calcium Homeostasis and Mitochondrial Dynamics in Parkinson's Disease. Antioxid Redox Signal 2019; Dec 1;31(16):1213-1234. PMID: 31303019
2018
Ugun-Klusek A, Theodosi TS, Fitzgerald JC, Burté F, Ufer C, Boocock D, Yu-Wai-Man P, Bedford L, Billett EE. Monoamine oxidase-A promotes protective autophagy in human SH-SY5Y neuroblastoma cells through Bcl-2 phosphorylation. Redox Biol. 2018; 20,167-181.PMID: 30336354
Jores T, Lawatscheck J, Beke V, Franz-Wachtel M, Yunoki K, Fitzgerald JC, Macek B, Endo T, Kalbacher H, Buchner J, and Rapaport D. Cytosolic Hsp70 and Hsp40 chaperones enable the biogenesis of mitochondrial β-barrel proteins. J. Cell. Biol. 2018; Sep 3;217(9):3091-3108. PMID: 29930205
Fitzgerald JC, Zimprich A, Bobbili DR, May P, Sharma M, Krüger R. Reply: No evidence for rare TRAP1 mutations influencing the risk of idiopathic Parkinson’s disease. Brain 2018; 141 (3):17. PMID: 29373630 * corresponding author
Sofi S, Fitzgerald JC, Jähn D, Dumoulin B, Stehling S, Kuhn H, Ufer C. Functional characterization of naturally occurring genetic variations of the human Guanine-rich RNA sequence binding factor 1 (GRSF1). Biochim. Biophys. Acta. 2018; 1862(4):866-876. PMID: 29366917
Marrone L, Bus C, Schöndorf D, Fitzgerald JC, Kübler M, et al. Generation of iPSCs carrying a common LRRK2 risk allele for in vitro modeling of idiopathic Parkinson's disease. PLOS One 2018; 13(3). PMID: 29513666
Rotermund C, Machetanz G, Fitzgerald JC. The Therapeutic Potential of Metformin in Neurodegenerative Diseases. Frontiers in Endocrinology 2018; 19;9:400. PMID:
Students
PhD
Lisa Schwarz, University of Tübingen (2018-), Katharina Stegen, University of Tübingen (2013-2018), Christine Bus, University of Tübingen (with Thomas Gasser, 2013-2018).
MSc Thesis
Lara-Sophie Rieder, University of Tübingen (2020), Orchid Ammar, University of Tübingen (2020), Kim Krieg, University of Constance (2018-2019), Max Mattheuer, MCBI, University of Tübingen (2018), Anna Schaedler, GTC, Tübingen (2018), Konstantina Kopolou, GTC, Tübingen (2017), Dilara Halim, GTC, Tübingen (2017), Benedikt Hoelbling, GTC, Tübingen (2017), Marco Siekmann, GTC, Tübingen (2017), Lisa Schwarz, GTC, Tübingen (2017).
BSc
Kevin Schindler, University of Tübingen (2015), Rahel Lewin, University of Tübingen (2014)














