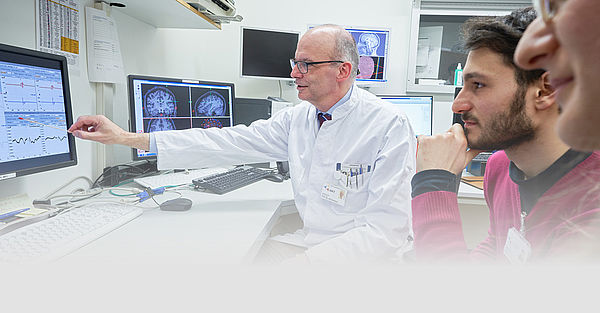
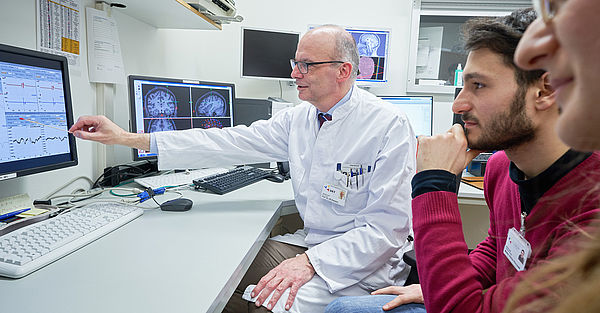
Personalized neurorehabilitative precision medicine – from data to therapies (Acronym: MWKNeuroReha)
Strokes is the most frequent cause of permanent disability. The present study investigates which factors contribute to how stroke patients benefit from neurorehabilitation by collecting clinical, electrophysiological, imaging and laboratory data in the acute phase of stroke as well as later during rehabilitation and after 90 days.
For each patient with acute stroke affecting one upper extremity, data is collected consisting of clinical tests to describe the impairment and the severity of stroke, structural and functional magnet resonance imaging, motor evoked potentials, electroencephalography, and laboratory workup. On admission to one of the cooperating neurorehabilitation hospitals and at intervals of 14 days, impairment and disability are tested again and added to the data set of each patient to quantify longitudinally stroke recovery. Moreover, during the inpatient period in the neurorehabilitation hospitals, the amount and types of neurorehabilitative treatments are recorded. 90 days after stroke, impairment of the affected upper extremity is measured (primary endpoint), furthermore range of activity of the upper extremity, dependency in daily life and quality of life is assessed (secondary endpoints).
Following a closed-loop approach, the data is then analyzed by a machine learning algorithm to create a personalized neurorehabilitation strategy.
This study is funded by “Forum Gesundheitsstandort Baden-Württemberg” of the “Ministerium für Wissenschaft, Forschung und Kunst Baden-Württemberg“.
Link: Study protocol MWKNeuroReha
Email: NeuroRehamed.uni-tuebingen.de

ConnectToBrain
ConnectToBrain will introduce whole-brain multi-locus transcranial magnetic stimulation (mTMS), in which the brain-stimulating electric-field location, direction, magnitude and timing are controlled electronically based on real-time high-density electroencephalography (hdEEG) information of activity and connectivity in brain networks.
Superpositions of electric fields produced by the different overlapping coils allow spatiotemporally millimeter- and millisecond-precise stimulus sequences to arbitrary cortical sites without physical movements of the coil set. Spatial targeting of mTMS will be further improved by measuring individual brain conductivity distributions with ultra-low-field MRI.
ConnectToBrain will allow unprecedented tracking of dynamic changes and reorganization of brain networks in real time, and network-targeted closed-loop stimulation. This radically novel technology will cause a paradigm shift from current open-loop practice that is only moderately effective in therapy. We will apply ConnectToBrain to reach new levels of efficacy of therapeutic applications. Patients after stroke and with Alzheimer’s disease will be tested and treated as models of network disorders.
Our high-risk, high-gain endeavor will reach the ambitious goals only through the synergy of 3 Principal Investigators, world leaders in their complementary areas of expertise (instrumentation, algorithms, translation): Prof. Risto Ilmoniemi, Prof. Gian Luca Romani, and Prof. Ulf Ziemann. If ConnectToBrain succeeds, we expect the value of societal, health and industrial benefits in Europe to exceed €1 billion annually, not to mention the immense value of alleviating human suffering from brain disorders
For more details on the ConnectToBrain initiative, please visit: https://www.connecttobrain.eu/

PHARMACOLOGICAL MODULATION OF TMS-EVOKED EEG RESPONSES
The ability to investigate brain function in healthy subjects as well as in patient groups with specific brain damage can be significantly improved by combining transcranial magnetic stimulation (TMS) with other electrophysiological and imaging methods, or by combining TMS with pharmacological interventions. Especially the technical ability of concurrently recording neuronal activity through EEG, which enables us to examine the direct effects of TMS on brain networks with sufficient spatial and excellent temporal resolution. TMS-evoked potentials (TEP) following a single TMS pulse of the primary motor cortex can be recorded both at the locus of stimulation and in more distant cortical regions in the period of up to 300ms following the stimulus. The underlying physiological mechanisms of TEP are not yet well understood. In this project, the physiological mechanisms of TMS-evoked EEG-recordings via pharmacological modulation of GABA-ergic neurotransmission is characterized more closely.
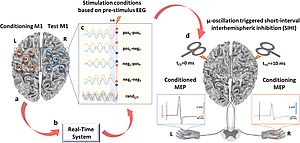
MODULATION OF THE CORTICAL MOTOR NEURONAL NETWORK THROUGH MULTI-COIL-TMS
Coordinated neuronal activity in large distributed brain networks is the basis for higher cognitive functions and complex sensorimotor abilities.
Within the motor functional system, numerous areas of the brain, such as the dorsal pre-motor cortex, the ventral pre-motor cortex, the supplementary motor area, the parietal cortex, the basal ganglia and the cerebellum, together with the primary motor cortex, constitute the motor network.
Neuronal coordination is highly dynamic and depends both on the motor task and on the condition of the brain. The effective connectivity in the brain's motor network after brain damage (e.g. following a stroke) is significantly altered. Therapeutic success in regaining motor abilities depends decisively on the correlation of changes in the neuronal network architecture with changes in motor abilities. In this project we use multi-coil-TMS in order to strategically modify effective connectivity in the cortical motor network and in order to study the effects on motor abilities and learning.
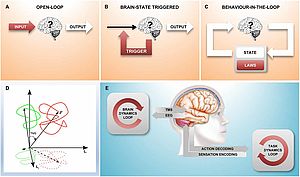
CLOSED-LOOP-STIMULATION
We are studying the relationship between the instantaneous state of cognitive processes and cortical information processing. A simultaneous EEG-TMS setup with real-time signal analysis allows EEG-controlled stimulus-pulses with deterministic latencies of less than 3 ms.
Our initial experiments examine the influence of the oscillatory phase of cortical EEG-alpha activity during the TMS-pulse on cortico-spinal excitability and plasticity. In addition to alpha, we currently have projects on theta oscillation-triggered stimulation.
Within the scope of a translational research project we are developing, based on this approach, new and therapeutically more effective, EEG-dependent TMS protocols for neuro-rehabilitation for stroke and Alzheimer’s disease.

Virtual-Reality-Based Motor Rehabilitation System: REHALITY
Ongoing brain dynamics predict behavior; brain computer interfaces (BCI) utilizing machine learning classifiers are increasingly accurate at predicting upcoming movement. There is clear potential to apply these advances to increase the effectiveness of personalized therapy for neurological dysfunction, but there is also a gap between what is possible in academic versus clinical contexts.
We aim to optimize therapy with respect to the constraints of clinical applications, while also creating an environment which is engaging and effective for the patient. By utilizing virtual reality, we are able to quite literally create the world within which our patient will be interacting. This means we can promote rehabilitative behaviors, all the while actively collecting data and adapting our algorithms in the background to continually give the best therapy possible. We lean on EEG-based BCIs to help with imaginary tasks of movement as found in “mirror therapy”, and utilize standard adaptive learning techniques to help widen movement arcs and strengthen areas of weakness.
In the end, our system will not only personalize to each individual, but it will adapt and grow with them as they progress on their rehabilitation path.
ConnectToBrain
ConnectToBrain will introduce whole-brain multi-locus transcranial magnetic stimulation (mTMS), in which the brain-stimulating electric-field location, direction, magnitude and timing are controlled electronically based on real-time high-density electroencephalography (hdEEG) information of activity and connectivity in brain networks.
Superpositions of electric fields produced by the different overlapping coils allow spatiotemporally millimeter- and millisecond-precise stimulus sequences to arbitrary cortical sites without physical movements of the coil set. Spatial targeting of mTMS will be further improved by measuring individual brain conductivity distributions with ultra-low-field MRI.
ConnectToBrain will allow unprecedented tracking of dynamic changes and reorganization of brain networks in real time, and network-targeted closed-loop stimulation. This radically novel technology will cause a paradigm shift from current open-loop practice that is only moderately effective in therapy. We will apply ConnectToBrain to reach new levels of efficacy of therapeutic applications. Patients after stroke and with Alzheimer’s disease will be tested and treated as models of network disorders.
Our high-risk, high-gain endeavor will reach the ambitious goals only through the synergy of 3 Principal Investigators, world leaders in their complementary areas of expertise (instrumentation, algorithms, translation): Prof. Risto Ilmoniemi, Prof. Gian Luca Romani, and Prof. Ulf Ziemann. If ConnectToBrain succeeds, we expect the value of societal, health and industrial benefits in Europe to exceed €1 billion annually, not to mention the immense value of alleviating human suffering from brain disorders
For more details on the ConnectToBrain initiative, please visit: https://www.connecttobrain.eu/
PHARMACOLOGICAL MODULATION OF TMS-EVOKED EEG RESPONSES
The ability to investigate brain function in healthy subjects as well as in patient groups with specific brain damage can be significantly improved by combining transcranial magnetic stimulation (TMS) with other electrophysiological and imaging methods, or by combining TMS with pharmacological interventions. Especially the technical ability of concurrently recording neuronal activity through EEG, which enables us to examine the direct effects of TMS on brain networks with sufficient spatial and excellent temporal resolution. TMS-evoked potentials (TEP) following a single TMS pulse of the primary motor cortex can be recorded both at the locus of stimulation and in more distant cortical regions in the period of up to 300ms following the stimulus. The underlying physiological mechanisms of TEP are not yet well understood. In this project, the physiological mechanisms of TMS-evoked EEG-recordings via pharmacological modulation of GABA-ergic neurotransmission is characterized more closely.
MODULATION OF THE CORTICAL MOTOR NEURONAL NETWORK THROUGH MULTI-COIL-TMS
Coordinated neuronal activity in large distributed brain networks is the basis for higher cognitive functions and complex sensorimotor abilities.
Within the motor functional system, numerous areas of the brain, such as the dorsal pre-motor cortex, the ventral pre-motor cortex, the supplementary motor area, the parietal cortex, the basal ganglia and the cerebellum, together with the primary motor cortex, constitute the motor network.
Neuronal coordination is highly dynamic and depends both on the motor task and on the condition of the brain. The effective connectivity in the brain's motor network after brain damage (e.g. following a stroke) is significantly altered. Therapeutic success in regaining motor abilities depends decisively on the correlation of changes in the neuronal network architecture with changes in motor abilities. In this project we use multi-coil-TMS in order to strategically modify effective connectivity in the cortical motor network and in order to study the effects on motor abilities and learning.
CLOSED-LOOP-STIMULATION
We are studying the relationship between the instantaneous state of cognitive processes and cortical information processing. A simultaneous EEG-TMS setup with real-time signal analysis allows EEG-controlled stimulus-pulses with deterministic latencies of less than 3 ms.
Our initial experiments examine the influence of the oscillatory phase of cortical EEG-alpha activity during the TMS-pulse on cortico-spinal excitability and plasticity. In addition to alpha, we currently have projects on theta oscillation-triggered stimulation.
Within the scope of a translational research project we are developing, based on this approach, new and therapeutically more effective, EEG-dependent TMS protocols for neuro-rehabilitation for stroke and Alzheimer’s disease.
Virtual-Reality-Based Motor Rehabilitation System: REHALITY
Ongoing brain dynamics predict behavior; brain computer interfaces (BCI) utilizing machine learning classifiers are increasingly accurate at predicting upcoming movement. There is clear potential to apply these advances to increase the effectiveness of personalized therapy for neurological dysfunction, but there is also a gap between what is possible in academic versus clinical contexts.
We aim to optimize therapy with respect to the constraints of clinical applications, while also creating an environment which is engaging and effective for the patient. By utilizing virtual reality, we are able to quite literally create the world within which our patient will be interacting. This means we can promote rehabilitative behaviors, all the while actively collecting data and adapting our algorithms in the background to continually give the best therapy possible. We lean on EEG-based BCIs to help with imaginary tasks of movement as found in “mirror therapy”, and utilize standard adaptive learning techniques to help widen movement arcs and strengthen areas of weakness.
In the end, our system will not only personalize to each individual, but it will adapt and grow with them as they progress on their rehabilitation path.
REORGANISATION OF CEREBRAL NETWORKS IN MULTIPLE SCLEROSIS
In multiple sclerosis (MS), multiple inflammatory CNS-lesions potentially interrupting cerebral networks to a significant degree. In contrast to an acute lesion of the brain as caused by a stroke, these lesions develop over time, allowing the brain more time to reorganize itself and to compensate for losses of ability.
Nevertheless, already at an early stage of the disease, cognitive functions such as attention and memory are frequently impaired.
Within the scope of this project, changes in the cortical networks of MS-patients are visualized with the help of fMRT and MEG and their influence on cognitive functions is studied.
These investigations may yield a neuro-biological characterisation of the immunomodulatory effects of drugs and the identification of biomarkers for monitoring the success of therapeutic interventions.

Local Partnerships
Prof. Christoph Braun
MEG-Zentrum, Universitätsklinikum Tübingen
Prof. Alireza Gharabaghi, Dr. Paolo Belardinelli
Klinik für Neurochirurgie, Universitätsklinikum Tübingen
Prof. Klaus Scheffler
Max-Planck-Institut für biologische Kybernetik
Prof. Cornelius Schwarz
Center for Integrative Neuroscience (CIN)
Prof. Markus Siegel
MEG-Zentrum, Universitätsklinikum Tübingen
National Partnerships
Dipl. Psych. Daniel Scholz, Prof. Eckart Altenmüller
Institut für Musikphysiologie und Musikermedizin, Hochschule für Musik, Theater und Medien, Hannover
Dr. Andreas Vlachos, Dr. Peter Jedlicka, Prof. Thomas Deller
Institut für Klinische Neuroanatomie, Goethe-Universität Frankfurt
International Partnerships
Prof. Vincenzo di Lazzaro
Institute of Neurology, Campus BioMedico University, Rom, Italien
Prof. John Rothwell
Sobell Department of Motor Neuroscience and Movement Disorders, UCL Institute of Neurology, Queen Square, London WC1N 3BG, United Kingdom
Prof. Hartwig Siebner, Prof. Axel Thielscher
Danish Research Centre for Magnetic Resonance, Center for Functional and Diagnostic Imaging and Research, Copenhagen University Hospital Hvidovre, Hvidovre, Dänemark
Dr. Cathy Stinear, Prof. Winston Byblow
Department of Sport and Exercise Science and Centre for Brain Research, University of Auckland, Neuseeland
BNP lab alumni
Til Ole Bergmann
Group Leader, AG Neurostimulation | Leibniz Institute for Resilience Research (LIR), Mainz, Germany
Ghazal Darmani
Postdoctoral fellow, Krembil Brain Institute | Toronto Western Hospital and University Health Network (UHN)
Debora Desideri
Former role: PhD student
Hanna Faber
Franca-Sophie König
Former role: Medical doctoral student
Semjon Levertov
Chen Liang
Former role: Medical doctoral student
Isabella Premoli
Postdoctoral fellow | King’s College London
Jakob Spogis
Former role: Medical doctoral student
Mohamed Yasser Elnaggar
Former role: Resident/Phd student
Carl Zipser
Local Partnerships
Prof. Christoph Braun
MEG-Zentrum, Universitätsklinikum Tübingen
Prof. Alireza Gharabaghi, Dr. Paolo Belardinelli
Klinik für Neurochirurgie, Universitätsklinikum Tübingen
Prof. Klaus Scheffler
Max-Planck-Institut für biologische Kybernetik
Prof. Cornelius Schwarz
Center for Integrative Neuroscience (CIN)
Prof. Markus Siegel
MEG-Zentrum, Universitätsklinikum Tübingen
National Partnerships
Dipl. Psych. Daniel Scholz, Prof. Eckart Altenmüller
Institut für Musikphysiologie und Musikermedizin, Hochschule für Musik, Theater und Medien, Hannover
Dr. Andreas Vlachos, Dr. Peter Jedlicka, Prof. Thomas Deller
Institut für Klinische Neuroanatomie, Goethe-Universität Frankfurt
International Partnerships
Prof. Vincenzo di Lazzaro
Institute of Neurology, Campus BioMedico University, Rom, Italien
Prof. John Rothwell
Sobell Department of Motor Neuroscience and Movement Disorders, UCL Institute of Neurology, Queen Square, London WC1N 3BG, United Kingdom
Prof. Hartwig Siebner, Prof. Axel Thielscher
Danish Research Centre for Magnetic Resonance, Center for Functional and Diagnostic Imaging and Research, Copenhagen University Hospital Hvidovre, Hvidovre, Dänemark
Dr. Cathy Stinear, Prof. Winston Byblow
Department of Sport and Exercise Science and Centre for Brain Research, University of Auckland, Neuseeland
BNP lab alumni
Til Ole Bergmann
Group Leader, AG Neurostimulation | Leibniz Institute for Resilience Research (LIR), Mainz, Germany
Ghazal Darmani
Postdoctoral fellow, Krembil Brain Institute | Toronto Western Hospital and University Health Network (UHN)
Debora Desideri
Former role: PhD student
Hanna Faber
Franca-Sophie König
Former role: Medical doctoral student
Semjon Levertov
Chen Liang
Former role: Medical doctoral student
Isabella Premoli
Postdoctoral fellow | King’s College London
Jakob Spogis
Former role: Medical doctoral student
Mohamed Yasser Elnaggar
Former role: Resident/Phd student
Carl Zipser
For the most updated publication list, please click here.
Gordon, P., Belardinelli, P., Stenroos, M., Ziemann, U., & Zrenner, C. (2022). Prefrontal theta phase-dependent rTMS-induced plasticity of cortical and behavioral responses in human cortex. Brain Stimulation, 15(2), 391-402. doi.org/10.1016/j.brs.2022.02.006
Metsomaa, J., Belardinelli, P., Ermolova, M., Ziemann, U., & Zrenner, C. (2021). Causal decoding of individual cortical excitability states. Neuroimage, 245, 118652. doi.org/10.1016/j.neuroimage.2021.118652
Humaidan, D., Vetter, D., Metsomaa, J., Ermolova, M., & Ziemann, U. (2021). Reinforcement machine learning for closed-loop rTMS stimulation of brain networks. Brain Stimulation, 14(6), 1696. doi.org/10.1016/j.brs.2021.10.346
Baur, D., Galevska, D., Hussain, S., Cohen, L., Ziemann, U., & Zrenner, C. (2020). Induction of LTD-like corticospinal plasticity by low-frequency rTMS depends on pre-stimulus phase of sensorimotor μ-rhythm. Brain Stimulation, 13(6), 1580-1587. doi.org/10.1016/j.brs.2020.09.005
Stefanou, M., Galevska, D., Zrenner, C., Ziemann, U., & Nieminen, J. (2020). Interhemispheric symmetry of µ-rhythm phase-dependency of corticospinal excitability. Scientific Reports, 10(1). doi.org/10.1038/s41598-020-64390-w
Zrenner, B., Zrenner, C., Gordon, P., Belardinelli, P., McDermott, E., & Soekadar, S. et al. (2020). Brain oscillation-synchronized stimulation of the left dorsolateral prefrontal cortex in depression using real-time EEG-triggered TMS. Brain Stimulation, 13(1), 197-205. doi.org/10.1016/j.brs.2019.10.007
Stefanou, M., Baur, D., Belardinelli, P., Bergmann, T., Blum, C., Gordon, P., Nieminen, J., Zrenner, B., Ziemann, U. and Zrenner, C., 2019. Brain State-dependent Brain Stimulation with Real-time Electroencephalography-Triggered Transcranial Magnetic Stimulation. Journal of Visualized Experiments, (150). doi: 10.3791/59711
Baur, D., Hussain, S., Cohen, L., Ziemann, U. and Zrenner, C., (2019). The influence of ongoing μ-oscillation phase on the induction of LTD-like plasticity with 1 Hz rTMS. Brain Stimulation, 12(2), p.565. DOI: doi.org/10.1016/j.brs.2018.12.872
Zrenner, B., Gordon, P., Kempf, A., Belardinelli, P., McDermott, E., Soekadar, S., Fallgatter, A., Zrenner, C., Ziemann, U. and Dahlhaus, F., (2019). Alpha-synchronized stimulation of the left DLPFC in depression using real-time EEG-triggered TMS. Brain Stimulation, 12(2), p.532. doi.org/10.1016/j.brs.2019.10.007
Zrenner, C., Desideri, D., Belardinelli, P., & Ziemann, U. (2018). Real-time EEG-defined excitability states determine efficacy of TMS-induced plasticity in human motor cortex. Brain Stimulation, 11(2), 374-389. doi.org/10.1016/j.brs.2017.11.016
For the most updated publication list, please click here.
2020
Bai, Y., Lin, Y., & Ziemann, U. (2020). Managing disorders of consciousness: the role of electroencephalography. Journal of neurology, 10.1007/s00415-020-10095-z. Advance online publication. https://doi.org/10.1007/s00415-020-10095-z
Ruiz-Gonzalez, Y., Velázquez-Pérez, L., Rodríguez-Labrada, R., Torres-Vega, R., & Ziemann, U. (2020). EMG Rectification Is Detrimental for Identifying Abnormalities in Corticomuscular and Intermuscular Coherence in Spinocerebellar Ataxia Type 2. Cerebellum (London, England), 19(5), 665–671. https://doi.org/10.1007/s12311-020-01149-z
Baur, D., Galevska, D., Hussain, S., Cohen, L. G., Ziemann, U., & Zrenner, C. (2020). Induction of LTD-like corticospinal plasticity by low-frequency rTMS depends on pre-stimulus phase of sensorimotor μ-rhythm. Brain stimulation, 13(6), 1580–1587. Advance online publication. https://doi.org/10.1016/j.brs.2020.09.005
Zrenner C, Galevska D, Nieminen JO, Baur D, Stefanou MI, Ziemann U. (2020). The shaky ground truth of real-time phase estimation [published online ahead of print, 2020 Mar 18]. Neuroimage;214:116761. doi:10.1016/j.neuroimage.2020.116761
Simmons, Z., & Ziemann, U. (2020). Terminology in neuromuscular electrodiagnostic medicine and ultrasound: Time for an update. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology, 131(7), 1655. https://doi.org/10.1016/j.clinph.2020.03.015
Lefaucheur, J. P., Aleman, A., Baeken, C., Benninger, D. H., Brunelin, J., Di Lazzaro, V., Filipović, S. R., Grefkes, C., Hasan, A., Hummel, F. C., Jääskeläinen, S. K., Langguth, B., Leocani, L., Londero, A., Nardone, R., Nguyen, J. P., Nyffeler, T., Oliveira-Maia, A. J., Oliviero, A., Padberg, F., … Ziemann, U. (2020). Corrigendum to "Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014-2018)" [Clin. Neurophysiol. 131 (2020) 474-528]. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology, 131(5), 1168–1169. https://doi.org/10.1016/j.clinph.2020.02.003
Ziemann U. (2020). I-waves in motor cortex revisited. Experimental brain research, 238(7-8), 1601–1610. https://doi.org/10.1007/s00221-020-05764-4
Rogasch, N. C., Zipser, C., Darmani, G., Mutanen, T. P., Biabani, M., Zrenner, C., Desideri, D., Belardinelli, P., Müller-Dahlhaus, F., & Ziemann, U. (2020). The effects of NMDA receptor blockade on TMS-evoked EEG potentials from prefrontal and parietal cortex. Scientific reports, 10(1), 3168. https://doi.org/10.1038/s41598-020-59911-6
Lefaucheur, J. P., Aleman, A., Baeken, C., Benninger, D. H., Brunelin, J., Di Lazzaro, V., Filipović, S. R., Grefkes, C., Hasan, A., Hummel, F. C., Jääskeläinen, S. K., Langguth, B., Leocani, L., Londero, A., Nardone, R., Nguyen, J. P., Nyffeler, T., Oliveira-Maia, A. J., Oliviero, A., Padberg, F., … Ziemann, U. (2020). Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014-2018). Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology, 131(2), 474–528. doi.org/10.1016/j.clinph.2019.11.002
2019
Bergmann, T. O., Lieb, A., Zrenner, C., & Ziemann, U. (2019). Pulsed Facilitation of Corticospinal Excitability by the Sensorimotor μ-Alpha Rhythm. The Journal of neuroscience : the official journal of the Society for Neuroscience, 39(50), 10034–10043. https://doi.org/10.1523/JNEUROSCI.1730-19.2019
Darmani G, Ziemann U (2019). Pharmacophysiology of TMS-evoked EEG potentials: A mini-review. Brain Stimul.;12(3):829-831. doi: 10.1016/j.brs.2019.02.021. Epub 2019 Feb 25
Ziemann U. (2019) Seventy years of our journal. <e>Clin Neurophysiol.;130(12):2255–2257. doi:10.1016/j.clinph.2019.10.006
<e>
Stefanou, M. I., & Ziemann, U. (2019). Neuroaesthetical Changes in Sculpture: The Case of Yannoulis Halepas (1851-1938). European neurology, 82(4-6), 116–123. https://doi.org/10.1159/000505546
Gasser, T., Lerche, H., & Ziemann, U. (2019). Personalisierte Diagnostik und Therapie in der Neurologie : Chance und Herausforderung [Personalized diagnostics and treatment in neurology : Prospect and challenge]. Der Nervenarzt, 90(8), 765–766. https://doi.org/10.1007/s00115-019-0757-8
Tabatabai, G., Tatagiba, M., Zips, D., & Ziemann, U. (2019). Neuroonkologie [Neuro-Oncology]. Der Nervenarzt, 90(6), 567. https://doi.org/10.1007/s00115-019-0732-4
Stefanou, M. I., Doycheva, D., Ebrahimi, A., Dörr, J. M., & Ziemann, U. (2019). Susac-Syndrom: ein diagnostisches Chamäleon [Susac Syndrome: A Diagnostic Chameleon]. Laryngo- rhino- otologie, 98(4), 268–275. https://doi.org/10.1055/a-0747-6916
Darmani, G., Bergmann, T. O., Zipser, C., Baur, D., Müller-Dahlhaus, F., & Ziemann, U. (2019). Effects of antiepileptic drugs on cortical excitability in humans: A TMS-EMG and TMS-EEG study. Human brain mapping, 40(4), 1276–1289. https://doi.org/10.1002/hbm.24448
2018
Zrenner C, Desideri D, Belardinelli P, Ziemann U (2018). Real-time EEG-defined excitability states determine efficacy of TMS-induced plasticity in human motor cortex. Brain Stimul.;11(2):374-389. doi: 10.1016/j.brs.2017.11.016. Epub 2017 Nov 24.
Stecker M, Pasqualetti P, Barry RJ, Daskalakis ZJ, Siebner HR, Ziemann U (2018). Reply to "is it significant? Is it relevant?" Clin Neurophysiol.;129(4):887. doi: 10.1016/j.clinph.2018.01.011. Epub 2018 Feb 2
Velázquez-Pérez L, Rodríguez-Labrada R, Torres-Vega R, Ortega-Sánchez R, Medrano-Montero J, González-Piña R, Vázquez-Mojena Y, Auburger G, Ziemann U (2018). Progression of corticospinal tract dysfunction in pre-ataxic spinocerebellar ataxia type 2: A two-years follow-up TMS study. Clin Neurophysiol.;129(5):895-900. doi: 10.1016/j.clinph.2018.01.066. Epub 2018 Mar 15.
Gordon PC, Zrenner C, Desideri D, Belardinelli P, Zrenner B, Brunoni AR, Ziemann U (2018). Modulation of cortical responses by transcranial direct current stimulation of dorsolateral prefrontal cortex: A resting-state EEG and TMS-EEG study. Brain Stimul.;11(5):1024-1032. doi: 10.1016/j.brs.2018.06.004.
Rodríguez-Labrada R, Velázquez-Pérez L, Ziemann U (2018). Transcranial magnetic stimulation in hereditary ataxias: Diagnostic utility, pathophysiological insight and treatment. Clin Neurophysiol.;129(8):1688-1698. doi: 10.1016/j.clinph.2018.06.003. Epub 2018 Jun 15.
Gordon PC, Desideri D, Belardinelli P, Zrenner C, Ziemann U (2018). Comparison of cortical EEG responses to realistic sham versus real TMS of human motor cortex. Brain Stimul.;11(6):1322-1330. doi: 10.1016/j.brs.2018.08.003. Epub 2018 Aug 16.
Stefanou, M. I., Desideri, D., Belardinelli, P., Zrenner, C., & Ziemann, U. (2018). Phase Synchronicity of μ-Rhythm Determines Efficacy of Interhemispheric Communication Between Human Motor Cortices. The Journal of neuroscience : the official journal of the Society for Neuroscience, 38(49), 10525–10534. doi:10.1523/JNEUROSCI.1470-18.201
Zipser, C. M., Premoli, I., Belardinelli, P., Castellanos, N., Rivolta, D., Heidegger, T., Müller-Dahlhaus, F., & Ziemann, U. (2018). Cortical Excitability and Interhemispheric Connectivity in Early Relapsing-Remitting Multiple Sclerosis Studied With TMS-EEG. Frontiers in neuroscience, 12, 393. https://doi.org/10.3389/fnins.2018.00393
Dietrich, S., Hertrich, I., Müller-Dahlhaus, F., Ackermann, H., Belardinelli, P., Desideri, D., Seibold, V. C., & Ziemann, U. (2018). Reduced Performance During a Sentence Repetition Task by Continuous Theta-Burst Magnetic Stimulation of the Pre-supplementary Motor Area. Frontiers in neuroscience, 12, 361. https://doi.org/10.3389/fnins.2018.00361
2017
Velázquez-Pérez L, Tünnerhoff J, Rodríguez-Labrada R, Torres-Vega R, Belardinelli P, Medrano-Montero J, Peña-Acosta A, Canales-Ochoa N, Vázquez-Mojena Y, González-Zaldivar Y, Auburger G, Ziemann U. (2017) Corticomuscular Coherence: a Novel Tool to Assess the Pyramidal Tract Dysfunction in Spinocerebellar Ataxia Type 2. Cerebellum;16(2):602-606. doi: 10.1007/s12311-016-0827-4.
Ziemann U (2017). Thirty years of transcranial magnetic stimulation: where do we stand? (2017) Exp Brain Res. 2017 Apr;235(4):973-984. doi: 10.1007/s00221-016-4865-4. Epub 2017 Jan 25.
Faber H, Opitz A, Müller-Dahlhaus F, Ziemann U (2017). Polarity-independent effects of tDCS on paired associative stimulation-induced plasticity. Brain Stimul.;10(6):1061-1069. doi: 10.1016/j.brs.2017.07.010. Epub 2017 Jul 27.
Stecker M, Pasqualetti P, Barry RJ, Daskalakis ZJ, Siebner HR, Ziemann U (2017). Statistical data analyses for clinical neurophysiology. Clin Neurophysiol.;128(10):1837-1838. doi: 10.1016/j.clinph.2017.07.396. Epub 2017 Jul 22.
Premoli I, Bergmann TO, Fecchio M, Rosanova M, Biondi A, Belardinelli P, Ziemann U (2017). The impact of GABAergic drugs on TMS-induced brain oscillations in human motor cortex. Neuroimage;163:1-12. doi: 10.1016/j.neuroimage.2017.09.023. Epub 2017 Sep 14.
Velázquez-Pérez L, Tünnerhoff J, Rodríguez-Labrada R, Torres-Vega R, Ruiz-Gonzalez Y, Belardinelli P, Medrano-Montero J, Canales-Ochoa N, González-Zaldivar Y, Vazquez-Mojena Y, Auburger G, Ziemann U (2017). Early corticospinal tract damage in prodromal SCA2 revealed by EEG-EMG and EMG-EMG coherence. Clin Neurophysiol.;128(12):2493-2502. doi: 10.1016/j.clinph.2017.10.009
Stefanou MI, Desideri D, Marquetand J, Belardinelli P, Zrenner C, Lerche H, Ziemann U. (2017). Motor cortex excitability in seizure-free STX1B mutation carriers with a history of epilepsy and febrile seizures. Clin Neurophysiol.;128(12):2503-2509. doi: 10.1016/j.clinph.2017.10.008. Epub 2017 Oct 20.
Ziemann U (2017). First virtual special issue (VSI) in Clinical Neurophysiology: A novel way of enhancing accessibility and visibility of published research. Clin Neurophysiol.;128(12):2527. doi: 10.1016/j.clinph.2017.11.001
2016
Ziemann U (2016). Clinical Neurophysiology--From present to future. Clin Neurophysiol.;127(1):1-2. doi: 10.1016/j.clinph.2015.10.048. Epub 2015 Nov 10.
2015
Murakami T, Kell CA, Restle J, Ugawa Y, Ziemann U. Left dorsal speech stream components and their contribution to phonological processing. J Neurosci 35:1411-1422
Fuhl A, Müller-Dahlhaus F, Lücke C, Tönnies SW, Ziemann U. Low doses of ethanol enhance LTD-like plasticity in human motor cortex. Neuropsychopharmacology, doi: 10.1038/npp.2015.151
Ziemann U, Siebner H. Inter-Subject and Inter-Session Variability of Plasticity Induction by Non-Invasive Brain Stimulation: Boon or Bane? Brain Stimulation 8: 662-663
2014
Ziemann U, Reis J, Schwenkreis P, Rosanova M, Strafella A, Badawy R, Muller-Dahlhaus FTMS and drugs revisited 2014
Clin Neurophysiol (doi: 10.1016/j.clinph.2014.08.028)
Di Pino G, Pellegrino G, Assenza G, Capone F, Ferreri F, Formica D, Ranieri F, Tombini M, Ziemann U, Rothwell JC, Di Lazzaro V
Modulation of brain plasticity in stroke: a novel model for neurorehabilitation
Nature reviews Neurology 10:597-608
Müller-Dahlhaus F, Ziemann U
Metaplasticity in Human Cortex
The Neuroscientist (doi: 10.1177/1073858414526645)
Goldsworthy MR, Müller-Dahlhaus F, Ridding MC, Ziemann U
Inter-subject Variability of LTD-like Plasticity in Human Motor Cortex: A Matter of Preceding Motor Activation
Brain Stimulation 7:864-870
Hamada M, Galea JM, Di Lazzaro V, Mazzone P, Ziemann U, Rothwell JC
Two distinct interneuron circuits in human motor cortex are linked to different subsets of physiological and behavioral plasticity
J Neurosci 34:12837-12849
Cash RF, Murakami T, Chen R, Thickbroom GW, Ziemann U
Augmenting Plasticity Induction in Human Motor Cortex by Disinhibition Stimulation
Cereb Cortex (doi: 10.1093/cercor/bhu176)
Lenz M, Platschek S, Priesemann V, Becker D, Willems LM, Ziemann U, Deller T, Muller-Dahlhaus F, Jedlicka P, Vlachos A
Repetitive magnetic stimulation induces plasticity of excitatory postsynapses on proximal dendrites of cultured mouse CA1 pyramidal neurons
Brain Structure & Function (10.1007/s00429-014-0859-9)
Premoli I, Rivolta D, Espenhahn S, Castellanos N, Belardinelli P, Ziemann U, Müller-Dahlhaus F Characterization of GABAB-receptor mediated neurotransmission in the human cortex by paired-pulse TMS-EEG
NeuroImage 103C:152-162.
Premoli I, Castellanos N, Rivolta D, Belardinelli P, Bajo R, Zipser C, Espenhahn S, Heidegger T, Müller-Dahlhaus F, Ziemann U
TMS-EEG signatures of GABAergic neurotransmission in the human cortex
J Neurosci 34:5603–5612
Gharabaghi A, Kraus D, Leao MT, Spüler M, Walter A, Bogdan M, Rosenstiel W, Naros G, Ziemann U
Coupling brain-machine interfaces with cortical stimulation for brain-state dependent stimulation: enhancing motor cortex excitability for neurorehabilitation
Front Hum Neurosci (8:122)
Lücke C, Heidegger T, Röhner M, Toennes SW, Krivanekova L, Müller-Dahlhaus F, Ziemann U
Deleterious effects of a low amount of ethanol on LTP-like plasticity in human cortex
Neuropsychopharmacol 39:1508-1518
Goldsworthy M, Müller-Dahlhaus F, Ridding M, Ziemann U
Resistant against de-depression: LTD-like plasticity in the human motor cortex induced by spaced cTBS
Cereb Cortex (doi:10.1093/cercor/bht353)
Grimaldi G, Argyropoulos GP, Boehringer A, Celnik P, Edwards MJ, Ferrucci R, Galea JM, Groiss SJ, Hiraoka K, Kassavetis P, Lesage E, Manto M, Miall RC, Priori A, Sadnicka A, Ugawa Y, Ziemann U
Non-invasive Cerebellar Stimulation - a Consensus Paper
Cerebellum 13(1):121-38
Rusu CV, Murakami M, Ziemann U, Triesch J
A model of TMS-induced I-waves in Motor Cortex
Brain Stim 7:401-414
2013
Kriváneková L, Baudrexel S, Bliem B, Ziemann U
Relation of brain stimulation induced changes in MEP amplitude and BOLD signal
Brain Stimul 6:330-9
Di Lazzaro V, Ziemann U
The contribution of transcranial magnetic stimulation in the functional evaluation of microcircuits in human motor cortex
Front Neural Circuits 7:18
Delvendahl I, Lindemann H, Heidegger T, Normann C, Ziemann U, Mall V
Effects of lamotrigine on human motor cortex plasticity
Clin Neurophysiol 124:148-53
2012
Vlachos A, Müller-Dahlhaus F, Rosskopp J, Lenz M, Ziemann U, Deller T
Repetitive magnetic stimulation induces functional and structural plasticity of excitatory postsynapses in mouse organotypic hippocampal slice cultures
J Neurosci 32:17514-23
Helfrich C, Pierau SS, Freitag CM, Roeper J, Ziemann U, Bender SMonitoring cortical excitability during repetitive transcranial magnetic stimulation in children with ADHD: a single-blind, sham-controlled TMS-EEG study PLoS One 7:e50073
Lu MK, Tsai CH, Ziemann U
Cerebellum to motor cortex paired associative stimulation induces bidirectional STDP-like plasticity in human motor cortex
Front Hum Neurosci 6:260
Murakami T, Müller-Dahlhaus F, Lu MK, Ziemann U
Homeostatic metaplasticity of corticospinal excitatory and intracortical inhibitory neural circuits in human motor cortex
J Physiol 590:5765-81
Restle J, Murakami T, Ziemann U
Facilitation of speech repetition accuracy by theta burst stimulation of the left posterior inferior frontal gyrus
Neuropsychologia 50:2026-31
Jung P, Klein JC, Wibral M, Hoechstetter K, Bliem B, Lu MK, Wahl M, Ziemann U
Spatiotemporal dynamics of bimanual integration in human somatosensory cortex and their relevance to bimanual object manipulation
J Neurosci 32:5667-77
Voytovych H, Kriváneková L, Ziemann U
Lithium: a switch from LTD- to LTP-like plasticity in human cortex.
Neuropharmacology 63:274-9
Hübers A, Klein JC, Kang JS, Hilker R, Ziemann U
The relationship between TMS measures of functional properties and DTI measures of microstructure of the corticospinal tract
Brain Stimul 5:297-304
Murakami T, Restle J, Ziemann U
Effective connectivity hierarchically links temporoparietal and frontal areas of the auditory dorsal stream with the motor cortex lip area during speech perception.
Brain Lang 122:135-41
Volz S, Nöth U, Jurcoane A, Ziemann U, Hattingen E, Deichmann R
Quantitative proton density mapping: correcting the receiver sensitivity bias via pseudo proton densities
Neuroimage 63:540-52
2011
Arai N, Müller-Dahlhaus F, Murakami T, Bliem B, Lu MK, Ugawa Y, Ziemann U
State-dependent and timing-dependent bidirectional associative plasticity in the human SMA-M1 network
J Neurosci 31:15376-83
Kriváneková L, Lu MK, Bliem B, Ziemann U
Modulation of excitability in human primary somatosensory and motor cortex by paired associative stimulation targeting the primary somatosensory cortex.
Eur J Neurosci 34:1292-300
Hattingen E, Magerkurth J, Pilatus U, Hübers A, Wahl M, Ziemann U
Combined (1)H and (31)P spectroscopy provides new insights into the pathobiochemistry of brain damage in multiple sclerosis
NMR Biomed 24:536-46
Korchounov A, Ziemann U
Neuromodulatory neurotransmitters influence LTP-like plasticity in human cortex: a pharmaco-TMS study
Neuropsychopharmacology 36:1894-902
Wahl M, Hübers A, Lauterbach-Soon B, Hattingen E, Jung P, Cohen LG, Ziemann U
Motor callosal disconnection in early relapsing-remitting multiple sclerosis
Hum Brain Mapp 32:846-55
Murakami T, Restle J, Ziemann U
Observation-execution matching and action inhibition in human primary motor cortex during viewing of speech-related lip movements or listening to speech
Neuropsychologia 49:2045-54
Lu MK, Arai N, Tsai CH, Ziemann U
Movement related cortical potentials of cued versus self-initiated movements: double dissociated modulation by dorsal premotor cortex versus supplementary motor area rTMS
Hum Brain Mapp 33:824-39
Kang JS, Terranova C, Hilker R, Quartarone A, Ziemann U
Deficient homeostatic regulation of practice-dependent plasticity in writer's cramp
Cereb Cortex 21:1203-12
2010
Heidegger T, Krakow K, Ziemann U
Effects of antiepileptic drugs on associative LTP-like plasticity in human motor cortex
Eur J Neurosci 32:1215-22
Szelényi A, Hattingen E, Weidauer S, Seifert V, Ziemann U
Intraoperative motor evoked potential alteration in intracranial tumor surgery and its relation to signal alteration in postoperative magnetic resonance imaging
Neurosurgery 67:302-13
Wagner M, du Mesnil de Rochemont R, Ziemann U, Hattingen E
Localization of thoracic CSF leaks by gadolinium-enhanced MR-myelography and successful MR-targeted epidural blood patching: a case report
J Neurol 257:1398-9
Lu MK, Jung P, Bliem B, Shih HT, Hseu YT, Yang YW, Ziemann U, Tsai CH
The Bereitschaftspotential in essential tremor
Clin Neurophysiol 121:622-30
2009
Alle H, Heidegger T, Kriváneková L, Ziemann U
Interactions between short-interval intracortical inhibition and short-latency afferent inhibition in human motor cortex
J Physiol 587:5163-76
Jung P, Ziemann U
Homeostatic and nonhomeostatic modulation of learning in human motor cortex
J Neurosci 29:5597-604
Lu MK, Bliem B, Jung P, Arai N, Tsai CH, Ziemann U
Modulation of preparatory volitional motor cortical activity by paired associative transcranial magnetic stimulation
Hum Brain Mapp 30:3645-56
Möller C, Arai N, Lücke J, Ziemann U
Hysteresis effects on the input-output curve of motor evoked potentials
Clin Neurophysiol 120:1003-8
2008
Peurala SH, Müller-Dahlhaus JFM, Arai N, Ziemann U
Interference of short-interval intracortical inhibition (SICI) and short-interval intracortical facilitation (SICF)
Clin Neurophysiol 119:2291-7
Hübers A, Orekhov Y, Ziemann U
Interhemispheric motor inhibition: its role in controlling electromyographic mirror activity
Eur J Neurosci 28:364-71
Müller-Dahlhaus JFM, Liu Y, Ziemann U
Inhibitory circuits and the nature of their interactions in the human motor cortex: a pharmacological TMS study
J Physiol 586:495-514
Müller-Dahlhaus JFM, Orekhov Y, Liu Y, Ziemann U
Interindividual variability and age-dependency of motor cortical plasticity induced by paired associative stimulation
Exp Brain Res 187:467-75
Bliem B, Müller-Dahlhaus JFM, Dinse HR, Ziemann U
Homeostatic metaplasticity in human somatosensory cortex
J Cogn Neurosci 20:1517-28
----------------------
Selected Review Papers (since 2008)
2014
Müller-Dahlhaus F, Ziemann U. Metaplasticity in human cortexNeuroscientist (doi: 10.1177/1073858414526645)
2013
Müller-Dahlhaus F, Vlachos A
Unraveling the cellular and molecular mechanisms of repetitive magnetic stimulation
Front Mol Neurosci 6: 50
2012
Nitsche MA, Müller-Dahlhaus F, Paulus W, Ziemann UThe pharmacology of neuroplasticity induced by non-invasive brain stimulation: building models for the clinical use of CNS active drugsJ Physiol 590:4641-62
2011
Ni Z, Müller-Dahlhaus F, Chen R, Ziemann U
Triple-pulse TMS to study interactions between neural circuits in human cortex
Brain Stim 4:281-93
Ziemann U, Wahl M, Hattingen E, Tumani H
Development of biomarkers for multiple sclerosis as a neurodegenerative disorder
Prog Neurobiol 95:670-85
Ziemann U
Transcranial magnetic stimulation at the interface with other techniques: a powerful tool for studying the human cortex
Neuroscientist 17:368-81
2010
Müller-Dahlhaus F, Ziemann U, Classen J
Plasticity resembling spike-timing dependent synaptic plasticity: the evidence in human cortex
Front Syn Neurosci 2:34
2008
Di Lazzaro V, Ziemann U, Lemon RN
State of the art: Physiology of transcranial motor cortex stimulation
Brain Stimul 1:345-62
Paulus W, Classen J, Cohen LG, Large CH, Di Lazzaro V, Nitsche M, Pascual-Leone A, Rosenow F, Rothwell JC, Ziemann U
State of the art: Pharmacologic effects on cortical excitability measures tested by transcranial magnetic stimulation
Brain Stimul 1:151-63

Hertie Center of Neurology
Hertie Institute for Clinical Brain Research
Department Neurology with Neurovascular Medicine
Hoppe-Seyler-Strasse 3
72076 Tübingen
Christine Riegraf
Tel.: +49 (0)7071 29-82049
Fax: +49 (0)7071 29-5260
christine.riegraf@med.uni-tuebingen.de
















